what do you do when you have no ehere to go
FDA Says "Do Not Get" COVID Vaccine If You Take This Condition
Those with "severe allergic reactions" should stay cautious, says the FDA.
March six, 2021
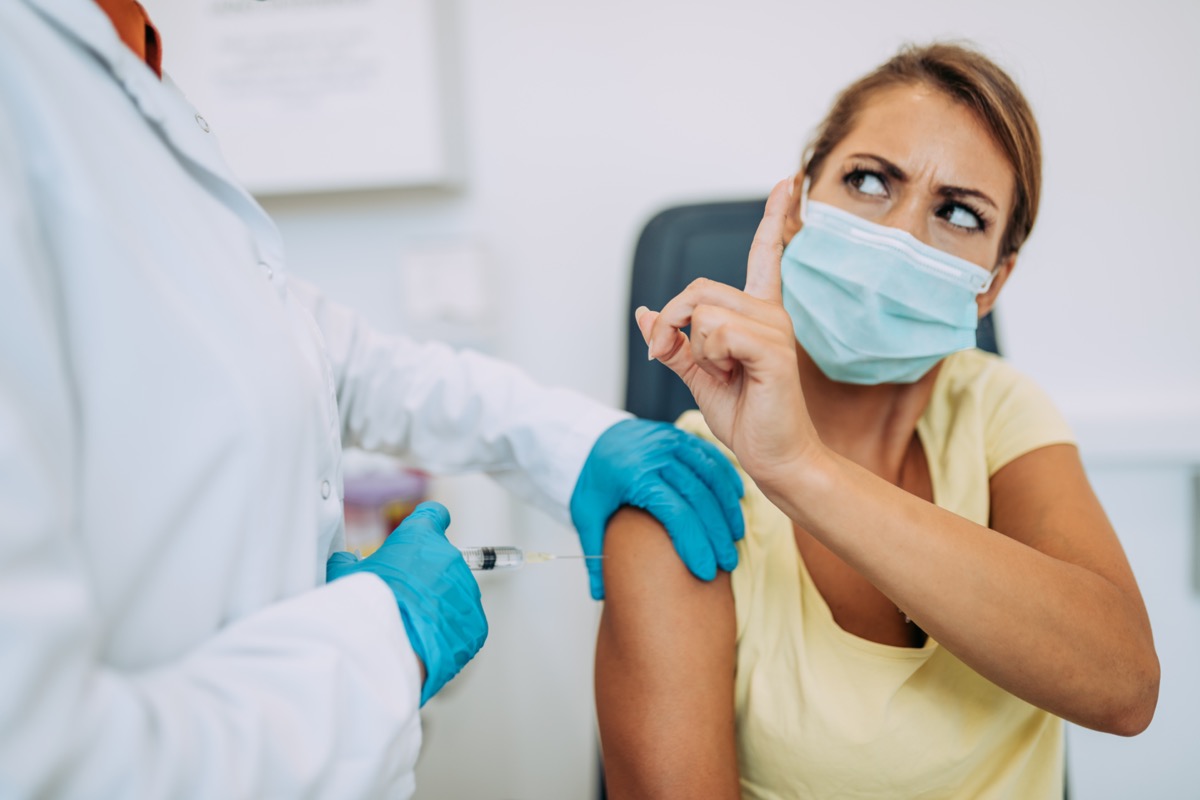
The Nutrient and Drug Assistants ( FDA ) approves the rubber of vaccines, and their feelings well-nigh the Pfizer vaccine are clear: "FDA evaluated and analyzed the safety and effectiveness data from clinical trials conducted in tens of thousands of study participants and manufacturing information submitted past Pfizer-BioNTech" and plant "articulate evidence that Pfizer-BioNTech COVID-19 Vaccine may be effective in preventing COVID-19 and support that the known and potential benefits outweigh the known and potential risks of the vaccine's use." So what are those risks? Read on to see who should not get the vaccine—and to ensure your health and the wellness of others, don't miss these Sure Signs Y'all've Already Had Coronavirus .

You may have heard that a small number of people had astringent allergic reactions to the Pfizer vaccine. "Severe allergic reactions, including anaphylaxis, have been reported following assistants of Pfizer-BioNTech COVID-nineteen Vaccine during mass vaccination outside of the clinical trial setting," says the FDA. Therefore: "You should non get the Pfizer-BioNTech COVID-19 Vaccine if y'all:
- had a severe allergic reaction afterwards a previous dose of this vaccine
- had a severe allergic reaction to whatever ingredient of this vaccine."
"What the Pfizer people are saying is that if y'all have a history of a astringent allergic reaction, you should either not take this vaccine, or if you practise have it, have it in the context of a place where if you practice develop an allergic reaction, information technology could be readily and effectively treated," said Dr. Anthony Fauci in a CNBC Salubrious Returns Livestream . Go along reading to run into what exactly is in the vaccine, to see if you might be allergic.

Says the FDA: "The Pfizer-BioNTech COVID-19 Vaccine includes the following ingredients: mRNA, lipids ((four-hydroxybutyl)azanediyl)bis(hexane-half dozen,1-diyl)bis(2-hexyldecanoate), 2 [(polyethylene glycol)-2000]-Northward,N-ditetradecylacetamide, 1,2-Distearoyl-sn-glycero-3- phosphocholine, and cholesterol), potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dihydrate, and sucrose." Next see what y'all should tell your vaccine administrator before getting yours.

Co-ordinate to the FDA, "tell the vaccination provider almost all of your medical conditions, including if you lot:
- have whatever allergies
- take a fever
- have a bleeding disorder or are on a blood thinner
- are immunocompromised or are on a medicine that affects your immune system
- are pregnant or plan to become pregnant
- are breastfeeding
- have received another COVID-19 vaccine."

The CDC has some skilful advice for those unsure: "If you accept had an firsthand allergic reaction—even if it was not severe—to a vaccine or injectable therapy for another disease, ask your md if you should get a COVID-19 vaccine. Your md will help you decide if it is safe for you to become vaccinated," they explain. Additionally, those with an allergy to polyethylene glycol (PEG) or polysorbate should also avoid getting it. "These recommendations include allergic reactions to PEG and polysorbate. Polysorbate is not an ingredient in either mRNA COVID-19 vaccine but is closely related to PEG, which is in the vaccines. People who are allergic to PEG or polysorbate should not get an mRNA COVID-xix vaccine," they explicate.
RELATED: Dr. Fauci Just Said When Nosotros'd Become Dorsum to Normal

The FDA says "serious adverse events, while uncommon (<ane.0%), were observed at slightly higher numerical rates in the vaccine study grouping compared to the saline placebo study group, both overall and for certain specific adverse events occurring in very modest numbers," says the FDA. "These represented common medical events that occur in the general population at like frequency. Upon further review by FDA, these imbalances do not raise a safety business, nor practise they propose a causal relationship to vaccination for the vast majority of reported serious adverse events.
Serious adverse events considered by FDA to be plausibly related to the vaccine or vaccination procedure were ane case of shoulder injury at the vaccination site and one case of swollen lymph node in the armpit opposite the vaccination arm."
Then barring whatever allergies, go vaccinated when it becomes available to y'all, and to protect your life and the lives of others, don't visit any of these 35 Places Y'all're Most Likely to Catch COVID .
Alek Korab is a Co-Founder and Managing Editor of the ETNT Health channel on Swallow This, Non That! Read more
Source: https://www.eatthis.com/news-fda-covid-vaccine-condition/
0 Response to "what do you do when you have no ehere to go"
Post a Comment